Blog By: Dr. Shivangi, True Value Dental Institute
Introduction
Mucormycosis or Black Fungus disease is an emerging angioinvasive infection caused by the ubiquitous filamentous fungi of the class of Zygomycetes (consisting of the orders Mucorales and Entomophthorales).1 The majority of human cases are caused by Mucorales fungi; therefore, the terms mucormycosis and zygomycosis are used interchangeably (the term phycomycosis is also used).2, 3 The most often involved Mucorales are those of the genera Rhizopus (the most common genus associated with mucormycosis), Lichtheimia (formerly known as Absidia and Mycocladus), and Mucor. Species of other Zygomycetes genera, such as Rhizomucor, Saksenaea, Cunninghamella, and Apophysomyces, are less common.3, 8 Mucorales species are vasotropic, causing tissue infarctions, and the mucormycosis spectrum ranges from cutaneous, rhinocerebral, and sinopulmonary to disseminated and frequent fatal infections, especially in immunocompromised hosts. 9, 10
The prevalence of mucormycosis in India is approximately 0.14 cases per 1000 population, about 80 times the prevalence in developed countries.11 COVID-19 infection has been associated with fungal infections.12 Mucormycosis is more often seen in immunocompromised individuals, and complications of orbital and cerebral involvement are likely in diabetic ketoacidosis and with concomitant use of steroids. The most common risk factor associated with mucormycosis is diabetes mellitus in India.13 In the background of the COVID-19 pandemic, only a limited number of cases of mucormycosis have been reported, but there are no known documented cases of sudden-onset visual loss with incidental COVID-19 infection in a newly detected young non-ketotic diabetic.14
Etiology
- Inhalation of fungal sporangiospores from air.15
- Direct inoculation of organisms into disrupted skin or mucosa.15
- Through decaying organic matter as they are also thermotolerant.1
- Seasonal variation à rhino-orbitocerebral mucormycosis (ROCM) in autumn season16 and pulmonary mucormycosis from August to Spetember.17
Predisposing Conditions and Risk Factors
- Hematological malignancies (HMs) with or without hematopoietic stem cell transplantation2
- Prolonged severe neutropenia
- Solid organ malignancies and solid organ transplantation (liver and heart) with dissemination of mucormycosis to distant organs occurred often after rejection and its treatment, preferentially to skin and soft tissues but not the brain.1
- Poorly controlled diabetes mellitus with or without diabetic ketoacidosis18
- Iron overload and chelation therapy with DFO (deferoxamine mesylate)
- Major trauma
- Prolonged use of corticosteroids and rheumatic diseases4 and a very high mortality if disseminated (88%)19
- Illicit intravenous drug use
- Neonatal prematurity and malnourishment
- Antifungal agents with no activity against Zygomycetes, such as voriconazole and caspofungin18, 20
- Hospital environment causing nosocomial mucormycosis21, 22 à associated with exposure to:
- heavy air fungal loads because of construction work,
- contaminated air filters,
- a variety of healthcare-associated procedures and devices, such as contaminated wound dressings, transdermal nitrate patches, intravenous catheters, tongue depressors, and even allopurinol pills
- Iatrogenic mini-outbreaks23, 24
- HIV or AIDS25
- Children à very rare à skin and gut affected more1
Covid-19 Disease Associated Mucormycosis (CAM)
Severe coronavirus disease (COVID-19) is currently associated with different types of opportunistic fungal infections including mucormycosis infections, ultimately leading to dissemination and death. While COVID-19 associated pulmonary aspergillosis is increasingly recognized, mucormycosis is rare. Mucormycosis is an uncommon but serious infection that complicates the course of severe COVID-19. A high index of suspicion and aggressive management is required to improve outcomes.
The primary reason that appears to be facilitating Mucorales spores to germinate in people with COVID-19 is26
- An ideal environment of low oxygen (hypoxia)
- COVID-19 (cytokine storm, lymphopenia, endothelial damage)
- High glucose (diabetes, new-onset hyperglycemia, steroid-induced hyperglycemia).27 It enhances the expression of glucose-regulator protein 78 (GRP-78) of endothelium cells and fungal ligand spore coating homolog (CotH) protein, enabling angioinvasion, hematogenous dissemination and tissue necrosis.28
- Acidic medium (metabolic acidosis, diabetic ketoacidosis [DKA])
- High iron levels (increased ferritins)
- Decreased phagocytic activity of white blood cells (WBC) due to immunosuppression (SARS-CoV-2 mediated, steroid-mediated or background comorbidities) coupled with several other shared risk factors including prolonged hospitalization with or without mechanical ventilators.
- Rampant use of systemic corticosteroids (increases blood glucose and opportunistic fungal infection)
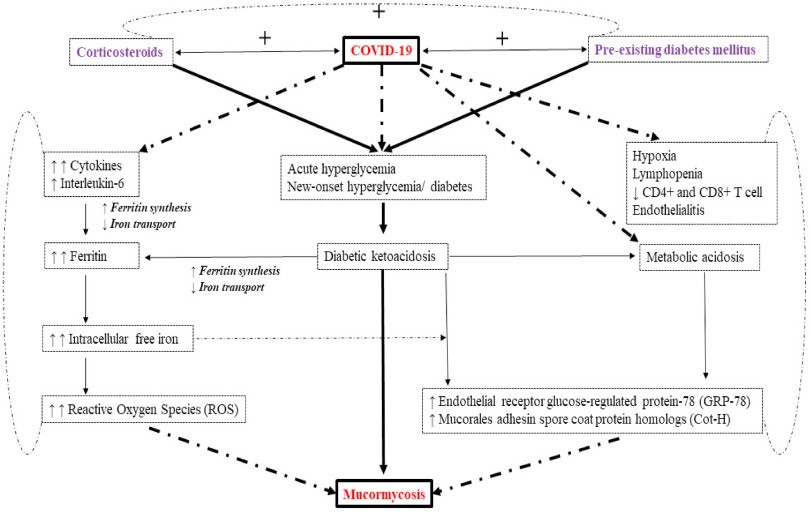
Figure 1: Postulated interaction of diabetes, corticosteroid and COVID-19 with mucormycosis26
Types of Mucormycosis
Based on its clinical presentation and anatomic site, invasive mucormycosis is classified into 6 major clinical forms as follows:29-31
- Rhinocerebral
- Pulmonary
- Cutaneous
- Gastrointestinal
- Disseminated
- Uncommon rare forms
Clinical Manifestations
The clinical hallmark of invasive mucormycosis is tissue necrosis resulting from angioinvasion and subsequent thrombosis. In most cases, the infection is rapidly progressive and results in death unless underlying risk factors (i.e., metabolic acidosis) are corrected and aggressive treatment with antifungal agents and surgical excision is instituted.1
| Type | Clinical manifestations |
| Pulmonary1 | Neutropenic patients undergoing chemotherapy32Mortality rate=76% and higher in severely immunosuppressed patients33Risk Factor à Diabetes mellitusHigh grade fever (>38oC) unresponsive to broad-spectrum antibiotics.Non-productive cough à commonHemoptysis, pleuritic chest pain, dyspnea à less commonEndobronchial or tracheal lesion à causes airway obstruction à lung collapse à invasion of hilar blood vessels with massive hemoptysis34-36 May invade lung-adjacent organs à mediastinum, pericardium, and chest wallIndistinguishable from pulmonary aspergillosis due to nonspecific signsAir crescent sign37, 38Multiple lung nodules and pleural effusion39 CT findings à A reversed halo sign, a focal round area of ground-glassattenuation surrounded by a ring of consolidation40 |
| Rhinocerebral | Most common form Risk factor à Diabetes mellitus18, 20, underlying malignancies, recipients of stem cell or solid organ transplants, and others41Develops after inhalation of fungal sporangiospores into the paranasal sinuses à infection rapidly spreads to adjacent tissuesInvades palate area posteriorly à to invade sphenoid sinus à laterally into cavernous sinus à orbits à brain42 (through orbital apex or cribriform plate of the ethmoid plate) à deathRarely causes hematogenous dissemination of the infection with or without development of mycotic aneurysms43Initial symptoms à sinusitis, periorbital cellulitis, eyes/facial pain, facial numbness, blurry vision4Diagnostic signs and symptoms à multiple cranial nerve palsies, unilateral periorbital facial pain, orbital inflammation, eyelid edema, blepharoptosis, proptosis, acute ocular motility changes, internal or external ophthalmoplegia, headache, and acute vision loss.Hallmark à A black necrotic eschar on faceFever is variable à may be absentElevated WBCs à typical signCT findings à edematous mucosa, fluid filling the ethmoid sinuses, destruction of periorbital tissues and bone margins44 |
| Cutaneous | Results from direct inoculation of fungal spores in the skin à leads to disseminated diseaseClassified as localized, deep extension and disseminated forms45Onset is gradual and progress slowly.May be fulminant à gangrene and hematogenous dissemination46-48A necrotic eschar with surrounding erythema and induration à may mimic pyoderma gangrenosum, bacterial synergistic gangrene, or other infections produced by bacteria or fungi49A nonspecific erythematous macule, small and insignificant à cutaneous manifestation of this disseminated disease47Less common clinical features à superficial lesions with only slightly elevated circinate and squamous borders resemblingtinea corporis45, targetoid plaques with outer erythematous rims and ecchymotic or blackened necrotic centers50, and, in patients with open wounds, lesions with a cottonlike appearance resembling that of bread mold51, 52 |
| Gastrointestinal | Uncommon and seldom diagnosed.Mortality rate (85%) is high delayed diagnosis.18Prevalence à premature neonates, malnourished children, HMs patients, diabetes mellitus, history of corticosteroid use53-58Source of infection à fermented milk, dried bread products15, fermented porridges, alcoholic drinks, spore-contaminated herbal and homeopathic remedies58, 59, sporangiospore contaminated tongue depressors54Affected areas à stomach (most common), colon, ileum57, 60, 61, liver, spleen and pancreas, bowel walls and blood vessels, bowel perforation, peritonitis, sepsis62, 63Clinical features à an appendiceal, cecal, or ileac mass or gastric perforation à with frequently massive upper gastrointestinal tract bleeding55, 58, 60, 61, 64-66, necrotizing enterocolitis in premature neonates, fever, typhlitis, hematochezia and a mass like appendiceal or ileal lesion in neutropenic patients4, 63 |
| Disseminated | Associated organs à lungs (most common), brain, liver, spleen, heart etc.67Medium à alimentary canal, burns, and extensive cutaneous lesionsRisk factors à patients with iron overload, profound immunosuppression or neutropenia and active leukemia3, 13, 67, 68A metastatic skin lesion à hallmark for early diagnosisIs always fatal if left without appropriate treatment69 |
| Uncommon form | Includes endocarditis, osteomyelitis, peritonitis, pyelonephritis a brain involvement.Risk factor à Intravenous drug use67, 71, 72 Endocarditis à principally on or around prosthetic valves àcause aortic thrombosis70Osteomyelitis à of tibia, cuboid, calcaneus, femur, humerus, scapula, metacarpals, phalanges, sternum74-77 à usually occurs after traumatic inoculation or surgical intervention (e.g., tibial pin placement, anterior cruciate ligament repair)71-73Hematogenous osteomyelitis àextremely rare77 Involvement of the peritoneal cavity by Zygomycetes in patients undergoing continuous ambulatory peritoneal dialysis à rare78, 79 |
Pathogenic Mechanism81
- Decreased phagocytic activity
- Accessible amounts of iron due to the displacement of protons by transferrin in diabetic ketoacidosis
- Fungal heme oxygenase, which promotes iron absorption for its metabolism
- A progressive increase in white blood cell count and neutrophils while lymphocytes progressively decreased.80
- Affects CD4+ and CD8+ T-cells, which are highly involved in the pathological process of COVID-19 infection.
- Reduction in the absolute number of lymphocytes and T-cells, which is associated with the worst outcomes.
- Mucorales-specific T-cells (CD4+ and CD8+) produce cytokines such as interleukin (IL) 4, IL-10, IL-17 and interferon-gamma (IFN-γ) that damage the fungal hyphae.
- An alteration in the T-cell population in COVID-19 infection is linked to the pathogenesis of fungal infection.
- Both mononuclear and polymorphonuclear phagocytes of normal hosts kill Mucorales by the generation of oxidative metabolites and the cationic peptides, defensins.82-84
- Exposure of neutrophils to R. oryzae hyphae results in up regulation in Toll-like receptor 2 expression and in a robust proinflammatory gene expression with rapid induction of NF-κB pathway–related genes.85
(Such specific T-cells were seen only in patients affected by invasive mucormycosis, and they concluded that they could be a useful surrogate diagnostic marker of an invasive fungal disease.)
Speculations:
- Lymphopenia could increase the risk of developing invasive mucormycosis.
- The recovery of lymphocyte count could improve the adaptive immune system and induce the production of Mucorales-specific T-cells à a role in controlling the invasive infection.
Oral Manifestations86
Starts as palatal ulceration or necrosis à occurs as a result of infection in the nasal cavity or paranasal sinuses.
↓
Facial cellulitis and anesthesia
↓
Nasal discharge
↓
Necrotic turbinate
↓
Fever, headache, and lethargy
↓
Eyes and orbit involvement
↓
Brain involvement
↓
Death – in severe cases
- Localized involvement of the mandibular gingiva and underlying bone.
- Attached gingiva buccal is grey and fibrotic in appearance and demonstrates a well-delineated sloughing border.
- A panoramic radiograph reveals horizontal bone loss and vertical defects.
- The palate, floor of the mouth, tongue, nasal mucosa. and nasal septum are unremarkable.
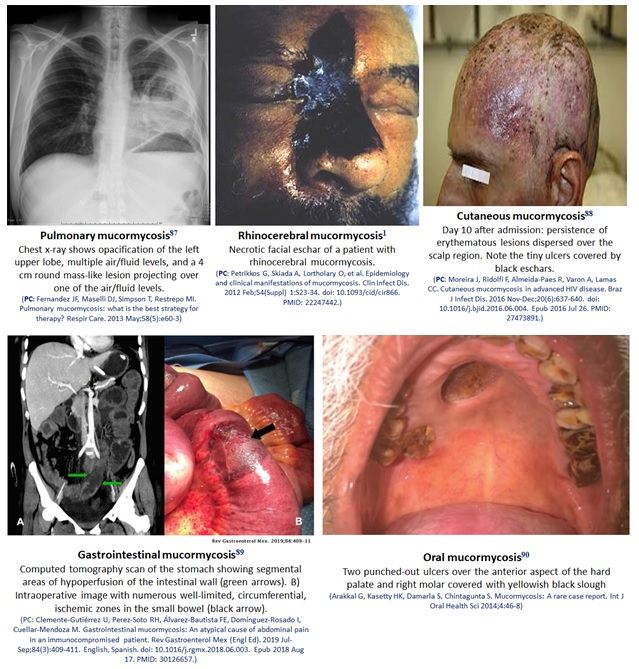
Figure 2: Different types of mucormycosis
Histopathology86
- Mucormycosis appears as broad (4 to 20 pm), non-septate hyphae that exhibit obtuse or right-angle branching. à diagnostic feature
- It is characterized by widespread tissue necrosis and an acute inflammatory infiltrate.
- The organisms have a predilection for invading blood vessels walls by direct extension and producing vascular thrombosis.
- Subsequently, the tissues supplied by the thrombosed vessels undergo ischemic necrosis.
- With time, lymphatic vessels and veins are also involved.91, 92
Oral Histopathology93
- Hard tissue reveals necrotic compact and cancellous bone trabeculae that exhibited irregular surface resorption.
- Masses of organisms are found withing the bone marrow spaces and resorptive crypts. They are elongated, broad, non-septate branching hyphae that contain terminal globose sporangia.
- Clusters of spores are seen.
- Soft tissue demonstrates mucosa, submucosa and attached bone fragments that contain a dense infiltrate of lymphocytes, plasma cells, and polymorphonuclear leukocytes. It also demonstrates necrotic stratified squamous epithelium and subjacent fibrous connective tissue.
- Numerous PAS-positive small oval spores are noted at the periphery of the specimen.
- The underlying fibrous connective tissue is composed of necrotic blood vessels and collagen fibers interspersed with fungal hyphae.

Figure 3: Histology of invasive mucormycosis94: (a) hematoxylin and eosin and (b) Grocott’s methenamine silver stain. Arrows indicate hyphal elements. (PC: Swaminathan S. Agents of Mucormycosis (Clinical Condition). Infectious Diseases. Available from: https://www.infectiousdiseaseadvisor.com/home/decision-support-in-medicine/infectious-diseases/agents-of-mucormycosis-clinical-condition/)
Investigative Approach95
- When the immune response is not able to control the initial infection, the spores germinate and invade the surrounding tissue.
- Initially, there is an edematous reaction, and by the time the hyphae invade blood vessels, the tissue becomes necrotic and acquires a characteristic black color.
- By contrast, immunocompetent individuals develop an intense inflammatory response and can present with a mass in the skin, respiratory sinuses, or the gastrointestinal tract.96
- Identification of these molds in tissues allows distinguishing the presence of the fungi as a pathogen from a culture contaminant and also is indispensable to define the presence of blood vessel affectation.
- Mucorales genera are characterized by nonpigmented, wide (5–20 μm), thin-walled, ribbon-like hyphae with some septations (pauciseptate) and a 90° angle branching.97
- The hyphae may vary in width, appear folded or crinkled, and be sparse or fragmented.
- In lesions exposed to air and in culture media, thick-walled spherical structures can form at the ends of the hyphae.
- Routine H&E stains may show only the cell wall without structure inside (Fig. 6.3e, f); in cytologic specimens, the hyphae can be identified using Papanicolaou and calcofluor white stains; and GMS and PAS can also help highlight the fungal wall.98
- In some situations, the hyphae may look degenerated, and many of the characteristics may not be appreciated in the specimen. In these cases, the pathologist must describe the degenerate hyphal elements observed in the specimen. This identifies the tissue where the fungus is found, ruling out the common possibility of culture contamination.
- In immunosuppressed patients, the hyphal elements are usually found immersed in abundant necrosis, hemorrhage, and blood vessel thrombosis.99
- Another important diagnostic feature is the identification of fungal elements invading the blood vessel wall or inside the lumen.
- Neutrophilic inflammation could also be identified surrounding the lesion.
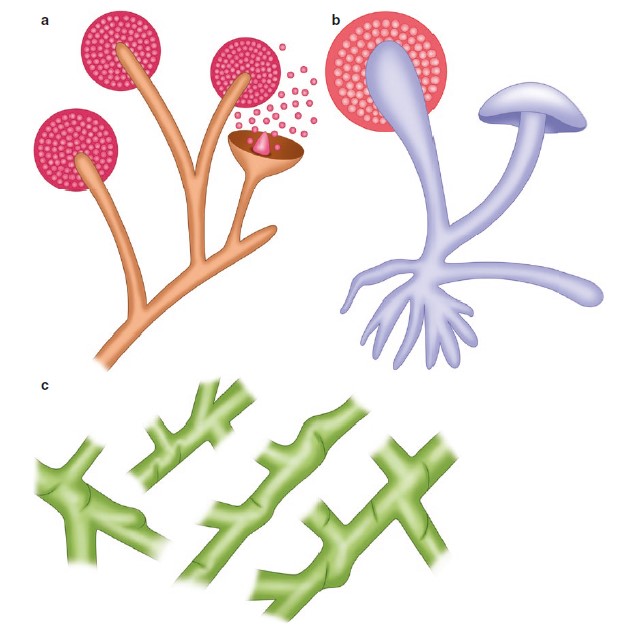
Figure 4:95 Mucorales. (a) Illustration, usual appearance of Mucor spp. (b) Illustration, usual appearance of Rhizopus spp. (c) Illustration, hyphae with some septations (pauciseptate) and a 90° angle branching.
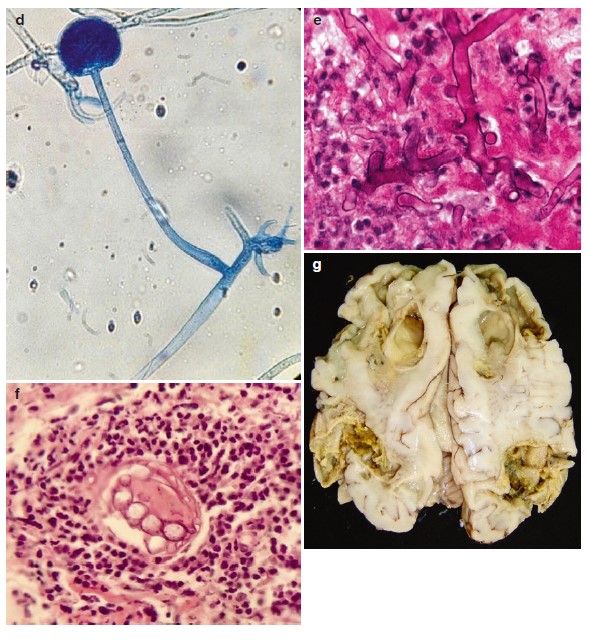
Figure 5:96 Mucorales.(d) Rhizopus spp. from culture. (e) H&E-stained specimen showing non-septate hyphae. (f) fungal elements invading the blood vessel wall or inside the lumen. (g) cerebral mucormycosis showing necrosis and tissue infarcts.
Differential Diagnosis100, 101
- Orbital cellulitis
- Cavernous sinus thrombosis
- Aspergillosis
- Nocardiosis
- Wegener’s granulomatosis
Diagnosis
The 1950 Smith and Krichner102 criteria for the clinical diagnosis of mucormycosis are still considered to be gold standard and include:
(i) Black, necrotic turbinate’s easily mistaken for dried, crusted blood,
(ii) Blood-tinged nasal discharge and facial pain, both on the same side,
(iii) Soft peri-orbital or peri-nasal swelling with discoloration and induration,
(iv) Ptosis of the eyelid, proptosis of the eyeball and complete ophthalmoplegia
(v) Multiple cranial nerve palsies unrelated to documented lesions.
Conclusion
Mucormycosis is a life-threatening infection that occurs in patients who are immunocompromised because of diabetic ketoacidosis, neutropenia, organ transplantation, and/or increased serum levels of available iron. Because of the increasing prevalence of diabetes mellitus, cancer, and organ transplantation, the number of patients at risk for this deadly infection is increasing. Moreover, individuals who lack phagocytes or have impaired phagocytic function are at higher risk of mucormycosis.103
Despite aggressive therapy, which includes disfiguring surgical debridement and frequently adjunctive toxic antifungal therapy, the overall mortality rate is high. New strategies to prevent and treat mucormycosis are urgently needed.
Following is the screening form for suspected rhino-cerebral mucormycosis for dental OPDs:

References
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012 Feb;54(Suppl) 1:S23-34. doi: 10.1093/cid/cir866. PMID: 22247442.
- Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005; 18:556–69.
- Prabhu RM, Patel R. Mucormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis and treatment. Clin Microbiol Infect. 2004; 10(Suppl 1):31–47.
- Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000; 13:236–301.
- Lass-Flo¨rl C. Zygomycosis: conventional laboratory diagnosis. Clin Microbiol Infect 2009; 15(Suppl 5):60–5.
- Kontoyiannis DP, Vartivarian S, Anaissie EJ, et al. Infections due to Cunninghamella bertholletiae in patients with cancer: report of three cases and review. Clin Infect Dis 1994; 18:925–8.
- Kwon-Chung KJ, Young RC, Orlando M. Pulmonary mucormycosis caused by Cunninghamella elegans in a patient with chronic myelogenous leukemia. Am J Clin Pathol 1975; 64:544–8.
- Ventura GJ, Kantarjian HM, Anaissie E, et al. Pneumonia with Cunninghamella species in patients with hematologic malignancies: a case report and review of the literature. Cancer 1986; 58:1534–6.
- Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi. 2020; 6:265.
- Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi. 2019; 5:26.
- Mehta S, Pandey A. Rhino-Orbital mucormycosis associated with COVID-19. Cureus. 2020; 12:e10726.
- Revannavar SM, P S S, Samaga L, et alCOVID-19 triggering mucormycosis in a susceptible patient: a new phenomenon in the developing world?BMJ Case Reports CP. 2021;14:e241663.
- Gonzalez CE, Rinaldi MG, Sugar AM. Mucormycosis. Infect Dis Clin North Am. 2002; 16:895–914, vi.
- Rogers TR. Treatment of mucormycosis: current and new options. J Antimicrob Chemother. 2008; 61(Suppl 1):35–9.
- Ibrahim A, Edwards JE Jr, Filler SG, eds. Mucormycosis. Philadelphia: Harcourt Brace, 2004.
- Talmi YP, Goldschmeid-Reouven A, Bakon M, et al. Rhino-orbital and rhino-orbitocerebral mucormycosis. Otolaryngol Head Neck Surg 2002; 127:22–31.
- Funada H, Matsuda T. Pulmonary mucormycosis in a hematology ward. Intern Med 1996; 35:540–4.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of mucormycosis: a review of 929 reported cases. Clin Infect Dis. 2005; 41:634–53.
- Shenoi S, Emery HM. Successful treatment of invasive gastric mucormycosis in a child with systemic lupus erythematosus. Lupus 2010;19:646–9.
- Skiada A, Pagano L, Groll A, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011; 17:1859–67.
- Perlroth J, Choi B, Spellberg B. Nosocomial fungal infections: epidemiology. Diagnosis and treatment. Med Mycol. 2007; 45:321–46.
- Petrikkos GL, Skiada A, Sambatakou H, et al. Mucormycosis: ten year experience in a tertiary-care centre in Greece. Eur J Clin Microbiol Infect Dis. 2003; 22:753–6.
- Antoniadou A. Outbreaks of mucormycosis in hospitals. Clin Microbiol Infect. 2009; 15(Suppl 5):55–9.
- Cheng VC, Chan JF, Ngan AH, et al. Outbreak of intestinal infection due to Rhizopus microsporus. J Clin Microbiol. 2009; 47:2834–43.
- Antinori S, Nebuloni M, Magni C, et al. Trends in the postmortem diagnosis of opportunistic invasive fungal infections in patients with AIDS: a retrospective study of 1630 autopsies performed between 1984 and 2002. Am J Clin Pathol. 2009; 132:221–7
- Singh AK, Singh R, Joshi SR, Misra A, Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India, Diabetes & Metabolic Syndrome: Clinical Research & Reviews (2021), doi: https://doi.org/10.1016/j.dsx.2021.05.019
- Garg D, Muthu V, Sehgal IS, Ramachandran R, Kaur H, Bhalla A, Puri GD, Chakrabarti A, Agarwal R. Coronavirus Disease (Covid-19) Associated Mucormycosis (CAM): Case Report and Systematic Review of Literature. Mycopathologia. 2021 May;186(2):289-298. doi: 10.1007/s11046-021-00528-2. Epub 2021 Feb 5. PMID: 33544266; PMCID: PMC7862973.
- Baldin C, Ibrahim AS. Molecular mechanisms of mucormycosis -The bitter and the sweet. PLoS Pathog 2017;13(8): e1006408.
- Goodman NL, Rinaldi MG. Agents of mucormycosis. In: Balows A, Hausler WJ, Herrmann KL, Isenberg HD, Shadoomy HJ, eds. Manual of clinical microbiology. 5th ed. Washington, DC: ASM Press, 1991:674–92.
- Lopes JO, Pereira DV, Streher LA, Fenalte AA, Alves SH, Benevenga JP. Cutaneous mucormycosis caused by Absidia corymbifera in a leukemic patient. Mycopathologia. 1995; 130:89–92.
- Stas KJF, Louwagie GLH, Van Damme BJC, Coosemans W, Waer M, Vanrenterghem YFC. Isolated mucormycosis in a bought living unrelated kidney transplant. Transplant Int. 1996; 9:600–2.
- Tedder M, Spratt JA, Anstadt MP, Hegde SS, Tedder SD, Lowe JE. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994; 57:1044–50.
- Torres-Narbona M, Guinea J, Martinez-Alarcon J, et al. Impact of mucormycosis on microbiology overload: a survey study in Spain. J Clin Microbiol. 2007; 45:2051–3.
- Gupta KL, Khullar DK, Behera D, Radotra BD, Sakhuja V. Pulmonary mucormycosis presenting as fatal massive haemoptysis in a renal transplant recipient. Nephrol Dial Transplant. 1998; 13:3258–60.
- Kitabayashi A, Hirokawa M, Yamaguchi A, Takatsu H, Miura AB. Invasive pulmonary mucormycosis with rupture of the thoracic aorta. Am J Hematol. 1998; 58:326–9.
- Passamonte PM, Dix JD. Nosocomial pulmonary mucormycosis with fatal massive hemoptysis. Am J Med Sci. 1985; 289:65–7.
- Dykhuizen RS, Kerr KN, Soutar RL. Air crescent sign and fatal haemoptysis in pulmonary mucormycosis. Scand J Infect Dis 1994; 26:498–501.
- Funada H, Misawa T, Nakao S, Saga T, Hattori KI. The air crescent sign of invasive pulmonary mucormycosis in acute leukemia. Cancer 1984; 53:2721–3.
- Chamilos G, Marom EM, Lewis RE, Lionakis MS, Kontoyiannis DP. Predictors of pulmonary mucormycosis versus invasive pulmonary aspergillosis in patients with cancer. Clin Infect Dis. 2005; 41:60–6.
- Wahba H, Truong MT, Lei X, Kontoyiannis DP, Marom EM. Reversed halo sign in invasive pulmonary fungal infections. Clin Infect Dis. 2008; 46:1733–7.
- Meyer RD, Rosen P, Armstrong D. Phycomycosis complicating leukemia and lymphoma. Ann Intern Med 1972; 77:871–9.
- Hosseini SM, Borghei P. Rhinocerebral mucormycosis: pathways of spread. Eur Arch Otorhinolaryngol. 2005; 262:932–8.
- Orguc S, Yuceturk AV, Demir MA, Goktan C. Rhinocerebral mucormycosis: perineural spread via the trigeminal nerve. J Clin Neurosci. 2005; 12:484–6.
- Franquet T, Gimenez A, Hidalgo A. Imaging of opportunistic fungal infections in immunocompromised patient. Eur J Radiol. 2004; 51:130–8.
- Oliveira-Neto MP, Da Silva M, Monteiro PCF, et al. Cutaneous mucormycosis in a young, immunocompetent girl. Med Mycol 2006; 44:567–70.
- Hampson FG, Ridgway EJ, Feeley K, Reilly JT. A fatal case of disseminated mucormycosis associated with the use of blood glucose self-monitoring equipment. J Infect. 2005; 51:e269-72.
- Hocker TL, Wada DA, Bridges A, El-Azhary R. Disseminated mucormycosis heralded by a subtle cutaneous finding. Dermatol Online J. 2010; 16:3.
- Rubin AI, Grossman ME. Bull’s-eye cutaneous infarct of mucormycosis: a bedside diagnosis confirmed by touch preparation. J Am Acad Dermatol. 2004; 51:996–1001.
- Kerr OA, Bong C, Wallis C, Tidman MJ. Primary cutaneous mucormycosis masquerading as pyoderma gangrenosum. Br J Dermatol. 2004; 150:1212–34.
- Rubin AI, Grossman ME. Bull’s-eye cutaneous infarct of mucormycosis: a bedside diagnosis confirmed by touch preparation. J Am Acad Dermatol. 2004; 51:996–1001.
- Chawla R, Sehgal S, Ravindra Kumar S, Mishra B. A rare case of mucormycosis of median sternotomy wound caused by Rhizopus arrhizus. Indian J Med Mycol. 2007; 25:419–21.
- Kordy FN, Al-Mohsen IZ, Hashem F, et al. Successful treatment of a child with posttraumatic necrotizing fasciitis caused by Apophysomyces elegans: case report and review of the literature. Pediatr Infect Dis J. 2004; 23:877–9.
- Diven SC, Angel CA, Hawkins HK, Rowen JL, Shattuck KE. Intestinal mucormycosis due to Absidia corymbifera mimicking necrotizing enterocolitis in a preterm neonate. J Perinatol. 2004; 24:794–6.
- Mitschell SD, Gray J, Morgan ME, et al. Nosocomial infection with Rhizopus microsporus in preterm infants: association with wooden tongue depressors. Lancet. 1996; 348:441–3.
- Michalak DM, Cooney DR, Rhodes KH, Telander RL, Kleinberg F. Gastrointestinal mucormycoses in infants and children: a cause of gangrenous intestinal cellulitis and perforation. J Pediatr Surg. 1980; 15:320–4.
- Garg PK, Gupta N, Gautam V, Hadke NS. Gastric mucormycosis: unusual cause of gastric perforation in an immunocompetent patient. South Med J. 2008; 101:449–50.
- Bittencourt AL, Ayala MA, Ramos EA. A new form of abdominal mucormycosis different from mucormycosis: report of two cases and review of the literature. Am J Trop Med Hyg. 1979; 28:564–9.
- Oliver MR, Van Voorhis WC, Boeckh M, et al. Hepatic mucormycosis in a bone marrow transplant recipient who ingested naturopathic medicine. Clin Infect Dis. 1996; 22:521–4.
- Ismail MH, Hodkinson HJ, Setzen G, Sofianos C, Hale MJ. Gastric mucormycosis. Trop Gastroenterol. 1990; 11:103–5.
- Geramizadeh B, Modjalal M, Nabai S, et al. Gastrointestinal mucormycosis: a report of three cases. Mycopathologia. 2007; 164:35–8.
- Echo A, Hovsepian RV, Shen GK. Localized cecal mucormycosis following renal transplantation. Transplant Infect Dis. 2005; 7:68–70.
- Virk SS, Singh RP, Arora AS, Grewal JS, Puri H. Gastric mucormycosis—an unusual cause of massive upper gastrointestinal bleed. Indian J Gastroenterol. 2004; 23:146–7.
- Cherney CL, Chutuape A, Fikrig MK. Fatal invasive gastric mucormycosis occurring with emphysematous gastritis: case report and literature review. Am J Gastroenterol 1999; 94:252–6.
- Azadeh B, McCarthy DO, Dalton A, Campbell F. Gastrointestinal mucormycosis: two case reports. Histopathology 2004; 44:298–300.
- Park YS, Lee JD, Kim TH, et al. Gastric mucormycosis. Gastrointest Endosc 2002; 56:904–5.
- Siu KL, Lee WH. A rare cause of intestinal perforation in an extreme low birth weight infant—gastrointestinal mucormycosis: a case report. J Perinatol. 2004; 24:319–21.
- Sanchez-Recalde A, Merino JL, Dominguez F, Mate I, Larrea JL, Sobrino JA. Successful treatment of prosthetic aortic valve mucormycosis. Chest 1999; 116:1818–20.
- McNab AA, McKelvie P. Iron overload is a risk factor for mucormycosis. Arch Ophthalmol. 1997; 115:919–21.
- Ingram CW, Sennesh J, Cooper JN, Perfect JR. Disseminated mucormycosis: report of four cases and review. Rev Infect Dis. 1989; 11:741–54.
- Kalayjian RC, Herzig RH, Cohen AM, Hutton MC. Thrombosis of the aorta caused by mucormycosis. South Med J. 1988; 81:1180–2.
- Burke WV, Zych GA. Fungal infection following replacement of the anterior cruciate ligament: a case report. J Bone Joint Surg Am. 2002; 84A:449–53.
- Pierce PP, Wood MB, Roberts GD, Fitzgerald RH, Robertson C, Edson RS. Saksenaea vasiformis osteomyelitis. J Clin Microbiol. 1987; 25:933–5.
- Holtom PD, Obuch AB, Ahlmann ER, Shepherd LE, Patzakis MJ. Mucormycosis of the tibia: a case report and review of the literature. Clin Orthop. 2000; 381:222–8.
- Wanishsawad C, Kimbrough RC, Chinratanalab S, Nugent K. Mucormycotic osteolytic rib lesion presenting as subacute pleural effusion. Clin Infect Dis. 1996; 22:715–16.
- Meis JF, Kullberg BJ, Pruszczynski M, Veth RP. Severe osteomyelitis due to the zygomycetes Apophysomyces elegans. J Clin Microbiol. 1994; 32:3078–81.
- Chaudhuri R, McKeown B, Harrington D,Hay RJ, Bingham JB, Spencer JD. Mucormycosis osteomyelitis causing avascular necrosis of the cuboid bone: MR imaging findings. Am J Roentgenol 1992; 159:1035–7.
- Echols RM, Selinger DS, Hallowell C, Goodwin JS, Duncan MH, Cushing AH. Rhizopus osteomyelitis. A case report and review. Am J Med. 1979; 66:141–5.
- Nayak S, Satish R, Gokulnath , Savio J, Rajalakshmi T. Peritoneal mucormycosis in a patient on CAPD. Perit Dial Int 2007; 27:216–17.
- Ram R, Swarnalatha G, Prasad N, Dakshinamurty KV. Exit site infection due to mucormycosis resulting in abdominal wall necrosis in a continuous ambulatory peritoneal dialysis patient. Nephrol Dial Transplant. 2007; 22:266–7.
- Pasero D, Sanna S, Liperi C, et al. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection 2020:1–6.
- Revannavar SM, P S S, Samaga L, et alCOVID-19 triggering mucormycosis in a susceptible patient: a new phenomenon in the developing world?BMJ Case Reports CP 2021;14:e241663.
- Waldorf AR, Ruderman N, Diamond RD. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. J Clin Invest 1984; 74:150–60.
- Waldorf AR. Pulmonary defense mechanisms against opportunistic fungal pathogens. Immunol Ser 1989; 47:243–71.
- Diamond RD, Haudenschild CC, Erickson NF 3rd. Monocyte-mediated damage to Rhizopus oryzae hyphae in vitro. Infect Immun 1982; 38:292–7.
- Chamilos G, Lewis RE, Lamaris G, Walsh TJ, Kontoyiannis DP. Zygomycetes hyphae trigger an early, robust proinflammatory response in human polymorphonuclear neutrophils through toll-like receptor 2 induction but display relative resistance to oxidative damage. Antimicrob Agents Chemother 2008; 52:722–4.
- Jones AC, Bentsen TY, Freedman PD. Mucormycosis of the oral cavity. Oral Surg Oral Med Oral Pathol. 1993 Apr;75(4):455-60. doi: 10.1016/0030-4220(93)90170-9. PMID: 8464609.
- Fernandez JF, Maselli DJ, Simpson T, Restrepo MI. Pulmonary mucormycosis: what is the best strategy for therapy? Respir Care. 2013 May;58(5):e60-3
- Moreira J, Ridolfi F, Almeida-Paes R, Varon A, Lamas CC. Cutaneous mucormycosis in advanced HIV disease. Braz J Infect Dis. 2016 Nov-Dec;20(6):637-640. doi: 10.1016/j.bjid.2016.06.004. Epub 2016 Jul 26. PMID: 27473891
- Clemente-Gutiérrez U, Perez-Soto RH, Álvarez-Bautista FE, Domínguez-Rosado I, Cuellar-Mendoza M. Gastrointestinal mucormycosis: An atypical cause of abdominal pain in an immunocompromised patient. Rev Gastroenterol Mex (Engl Ed). 2019 Jul-Sep;84(3):409-411. English, Spanish. doi: 10.1016/j.rgmx.2018.06.003. Epub 2018 Aug 17. PMID: 30126657
- Arakkal G, Kasetty HK, Damarla S, Chintagunta S. Mucormycosis: A rare case report. Int J Oral Health Sci 2014;4:46-8
- Lehrer RI, Howard DH, Sypherd PS, et al. Mucormycosis. Ann Intern Med. 1980; 93:93-108.
- Eisenberg L, Wood T, Boles R. Mucormycosis Laryngoscope 1977; 87:347-56.
- Jones AC, Bentsen TY, Freedman PD. Mucormycosis of the oral cavity. Oral Surg Oral Med Oral Pathol. 1993 Apr;75(4):455-60. doi: 10.1016/0030-4220(93)90170-9. PMID: 8464609.
- Swaminathan S. Agents of Mucormycosis (Clinical Condition). Infectious Diseases. Available from: https://www.infectiousdiseaseadvisor.com/home/decision-support-in-medicine/infectious-diseases/agents-of-mucormycosis-clinical-condition/
- Jurado LF, López-Panqueva RP. Histopathology. In: Turgut M., Challa S., Akhaddar A. (eds) Fungal Infections of the Central Nervous System. Springer, Cham. 2019; 51-73. https://doi.org/10.1007/978-3-030-06088-6_6
- Roilides E, Antachopoulos C, Simitsopoulou M. Pathogenesis and host defence against Mucorales: the role of cytokines and interaction with antifungal drugs. Mycoses. 2014;57(Suppl 3):40–7. https://doi.org/10.1111/myc.12236.
- Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000; 13:236–301.
- Naggie S, Perfect JR. Molds: hyalohyphomycosis, phaeohyphomycosis, and zygomycosis. Clin Chest Med. 2009; 30:337–53.
- Ben-Ami R, Luna M, Lewis RE, Walsh TJ, Kontoyiannis DP. A clinicopathological study of pulmonary mucormycosis in cancer patients: extensive angioinvasion but limited inflammatory response. J Infect. 2009; 59:134–8.
- Long B, Koyfman A. Mucormycosis: what emergency physicians need to know? Am J Emerg Med. 2015 Dec;33(12):1823-5
- Hernández JL, Buckley CJ. Mucormycosis. 2020 Jun 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–. PMID: 31335084.
- Smith HW, Kirchner JA Cerebral mucor-mycosis: A report of 3 cases. Arch Otolaryng (Chicago) 1950; 68:715-726
- Ibrahim A, Spellberg B, Walsh T, Kontoyiannis D. Pathogenesis of Mucormycosis. Clinical Infectious Diseases. May 28, 2012; 54, S16-S22.
Blog By: Dr. Shivangi, True Value Dental Institute

 Click here for Dental Services
Click here for Dental Services
